The medical device sector is crucial for providing quality healthcare to citizens. It is, therefore, an important player in both the European and global economies.
The EU’s medical devices sector is competitive and innovative and includes actively participating small and medium-sized enterprises.
The sector is supported by a medical device regulation framework whose aim is to ensure the smooth functioning of the internal market, with the primary focus being a high level of protection for patients and other users. More than 500,000 types of medical devices, (including In Vitro Diagnostics - IVDs) can be found on the EU market.
Medical Device: Definition
According to the WHO, a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material, or other similar or related article intended by the manufacturer to be used, alone or in combination, for a medical purpose.
Section 201(h) of the Food, Drug & Cosmetic Act (FD&C Act) of the U.S. FDA defines a medical device as an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory which is:
- recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease in man or other animals, or,
- intended to affect the structure or any function of the body of man or other animals,
- and does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.
- The term "device" does not include software functions excluded pursuant to section 520(o).
The EU Medical Device Regulation (MDR) defines a medical device as:
Any instrument, apparatus, appliance, software, implant, reagent, material, or other article intended by the manufacturer to be used, alone or in combination, for human beings for one or more of the following specific medical purposes:
- Diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease,
- Diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability,
- Investigation, replacement, or modification of the anatomy or of a physiological or pathological process or state,
- Providing information by means of in vitro examination of specimens derived from the human body, including organ, blood, and tissue donations, and which does not achieve its principal intended action by pharmacological, immunological, or metabolic means, in or on the human body, but which may be assisted in its function by such means.
The following products shall also be deemed to be medical devices:
- Devices for the control or support of conception.
- Products specifically intended for the cleaning, disinfection, or sterilization of devices as referred to in Article 1(4) and of those referred to in the first paragraph of this point.
This article might interest you ⤵️

Which medical devices fall under the MDR
Alongside this definition, Annex XVI of the MDR includes certain products that also explicitly fall within the scope of the MDR. These are products that do not have a medical purpose but are similar to medical devices.
Article 2 of the IVDR offers the following definition for an IVD medical device:
Any medical device that is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software, or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
- Physiological or pathological process or state
- Congenital physical or mental impairments
- The predisposition to a medical condition or a disease
- Determination of safety and compatibility with potential recipients
- Predicting treatment response or reactions
- Defining or monitoring therapeutic measures
- Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices,
Classification of Medical Devices
In the European Union’s medical device regulation process, it is necessary to first determine whether the device is classified under Medical Device Regulation (MDR) No. 2017/745 for medical devices, active implantable medical devices, or In Vitro Diagnostic Device Regulation (IVDR) No. 2017/746 for IVD devices. Note that the MDR also includes products specifically delineated in Annex XVI that do not have a medical purpose.
Europe adopts a rule-based classification scheme for medical devices. Annex VIII of the MDR now has 22 rules. The extent of regulatory control and MDR compliance requirements increase according to the level of risk associated with the finished medical device. The European system contrasts with that of the U.S. which is largely based on identifying similar devices (predicates) already cleared by the FDA.
How are medical devices classified in Europe?
According to MDR Article 51, devices are grouped into classes I, IIa, IIb, and III, taking into account the intended purpose of the devices and their inherent risks. Classification is based on Annex VIII of the MDR.
Classification is based on Annex VIII of the MDR. Additionally, Article 52(7)(a), (b), and (c), further subdivides Class I devices into Is – sterile condition, Im – measuring function, and Ir – reusable surgical.
- Class I – Provided non-sterile or lacks a measuring function (low risk)
- Class I – Provided sterile and/or includes a measuring function (low/medium risk); the MDR includes reusable surgical instruments (as Class I reusable surgical instruments) in this group.
Class I examples include corrective glasses, vehicles for disabled people, crutches, etc.
- Class IIa (medium risk)
Class IIa examples include contact lenses, ultrasound devices, dental crowns, etc
- Class IIb (medium/high risk)
Class IIb examples include condoms, lens disinfection products, etc
- Class III (high risk)
Class III examples include breast implants, stents, hip prostheses, etc.
Essentially, all devices belong to 4 basic categories:
- Non-invasive devices
- Invasive medical devices
- Active medical devices
- Special Rules (including contraceptive, disinfectant, and radiological diagnostic medical devices)
The MDR also includes a few additional special rules (e.g., one for nanomaterials).
IVD Classification
Regulation (EU) 2017/746 groups IVD medical devices into classes A, B, C, and D, taking into account the intended purpose of the devices and their inherent risks (Article 47).
The EU notes that robust risk-based classification rules are essential for the correct classification of devices (according to Annex VIII), as certain requirements set out by the IVDR are directly linked to device classification.
Particularly, the conformity assessment route highly depends on classification, which is reflected in concepts such as conformity assessment scrutiny of class D devices (Article 50); the involvement of European Union reference laboratories for high-risk devices (Article 100); and a consultation with a national medicines agency or the European Medicines Agency (EMA) for companion diagnostics (Article 48(3)).
Many post-market requirements are also class dependent, for instance, annual surveillance assessments for class C and D devices or the requirement to prepare a postmarket surveillance report or Periodic Safety Update Report (PSUR) for a given device (Articles 80 and 81).
Conditions for Placing a Medical Device on the Market
To be placed on the market in the EU, a medical device must comply with the safety and health requirements. The placing of a medical device on the market is conditional on obtaining the CE marking prior to its marketing. The marking reflects the conformity of the medical device with the safety and health requirements set out in European legislation.
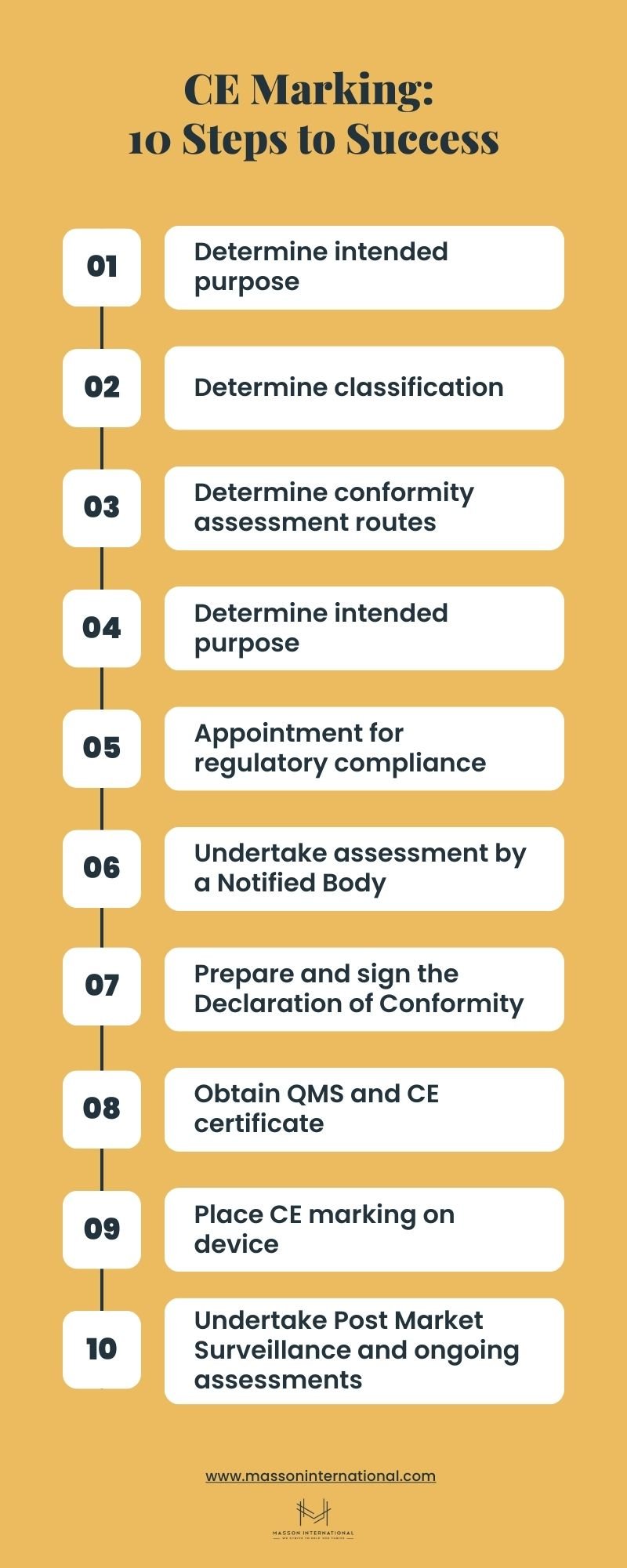
CE Marking: 10 Steps to Success
- Determine intended purpose
- Determine classification
- Determine conformity assessment routes
- Comply with conformity requirements
- Quality management system
- Technical documentation - Appoint a person responsible for regulatory compliance
- Undertake assessment by a Notified Body
- Prepare and sign the Declaration of Conformity
- Obtain QMS and CE certificate
- Place CE marking on device
- You’re done - Undertake Post Market Surveillance and ongoing assessments
You can read more about these steps here.
This article might interest you ⤵️
Applicable Legislations
The Regulations on Medical Devices (Regulation (EU) 2017/745) and on In Vitro Diagnostic Devices (Regulation (EU) 2017/746) changed the European legal framework for medical devices, ushering in new responsibilities for EMA and national competent authorities in the assessment of certain categories of medical devices.
The Medical Devices Regulation has been in effect since 26 May 2021. Manufacturers must comply with the Regulations when placing new medical devices on the market. It repeals Directive 93/42/EEC on medical devices and Directive 90/385/EEC on active implantable medical devices.
The In Vitro Diagnostic Devices Regulation applies since 26 May 2022. It repeals Directive 98/79/EC of the European Parliament and of the Council on in vitro diagnostic medical devices.
Frequently Asked Questions
What is the 2023 Regulation for Medical Devices?
On March 15, 2023, Regulation (EU) 2023/607 of the European Parliament and of the Council amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards the transitional provisions for certain medical devices and in vitro diagnostic medical devices were adopted in Europe.
Among other changes, paragraph 3 of Article 120 of (EU) 2017/745 has been replaced to include the provision that devices that have a certificate that was issued in accordance with Directive 90/385/EEC or Directive 93/42/EEC may be placed on the market or put into service until the following dates:
- 31 December 2027, (for all class III devices; and for class IIb implantable devices except sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips, and connectors)
- 31 December 2028, (for class IIb devices other than those covered in the above provision; for class IIa devices; and for class I devices placed on the market in sterile condition or having a measuring function)
- Devices for which the conformity assessment procedure pursuant to Directive 93/42/EEC did not require the involvement of a notified body, for which the declaration of conformity was drawn up prior to 26 May 2021 and for which the conformity assessment procedure pursuant to this Regulation requires the involvement of a notified body, may be placed on the market or put into service until 31 December 2028
Among other changes, paragraph 4 of Article 110 of Regulation (EU) 2017/746 is amended as follows:
- Devices lawfully placed on the market pursuant to Directive 98/79/EC prior to 26 May 2022, and devices lawfully placed on the market from 26 May 2022 pursuant to paragraph 3 of this Article may continue to be made available on the market or put into service.
What is the New EU Regulation for Medical Devices?
The following medical device Directives were repealed and replaced by Regulation (EU) 2017/746 and Regulation (EU) 2017/745 respectively:
- 1998: Directive 98/79/EC of the European Parliament and of the Council on In Vitro Diagnostic Medical Devices (IVDMD)
- 1993: Council Directive 93/42/EEC on Medical Devices (MDD)
- 1990: Council Directive 90/385/EE on Active Implantable Medical Devices (AIMDD)
Adopted in May 2017, the new rules will fully apply after the transitional periods provided for in the regulations. The transitional periods have since ended and the new EU Regulation applies from:
- 26 May 2021: Regulation (EU) 2017/745 on medical devices
- 26 May 2022: Regulation (EU) 2017/746 on in vitro diagnostic medical devices
What are the EU Standards for Medical Devices?
The harmonized standards for medical devices drafted in support of Regulation (EU) 2017/745 of the European Parliament and of the Council (Decision (EU) 2021/1182 of 16 July 2021) refers to standards such as:
- EN ISO 10993-23:2021
- EN ISO 11135:2014
- EN ISO 11137-1:2015
- EN ISO 11737-2:2020
- EN ISO 25424:2019
The harmonized standards for in vitro diagnostic medical devices drafted in support of Directive 98/79/EC of the European Parliament and of the Council (Decision (EU) 2020/439 of 24 March 2020) refers to standards such as:
- EN 556-1:2001
- EN 556-2:2015
- EN ISO 11137-1:2015
- EN ISO 11137-2:2015
- EN ISO 11737-2:2009
Although we are not a company with expertise in CE mark, CE-rep and Medical Device registration in Europe (or Switzerland), we have partner companies in our network we can introduce you to. Feel free to contact us for more details.
The information contained in this article and website is provided solely for general interest and may not reflect current legal developments and therefore should not be relied upon or construed as legal advice or expertise. We make no representations or warranties of any kind, express or implied, about the completeness, accuracy, reliability, suitability or availability with respect to the website or the information, products, services, or related graphics contained on the website for any purpose. Any reliance you place on such information is therefore strictly at your own risk.For more specific, comprehensive and up-to-date information, or for help with particular factual situations, you should seek the opinion of legal counsel and professionals in medical device registration.In no event will we be liable for any loss or damage including without limitation, indirect or consequential loss or damage, or any loss or damage whatsoever arising from loss of data or profits arising out of, or in connection with, the use of this article and website.




